For more on this topic, go to www.dentaleconomics.com and search using the following key words: periodontal disease, gingivitis, oral biofilms, Richard H. Nagelberg, DDS.
Unraveling the mechanisms involved in the development of periodontal disease has occurred over a relatively short period of time. Research continues to add pieces to the puzzle almost daily. For more than two decades starting in the early 1960s, prevailing wisdom held that all the bacteria in the mouth contribute to the development and progression of periodontal disease and that bacterial acids sever the gingival attachment. Home-care recommendations and treatments were developed with this consensus in mind. “WE NOW KNOW” are words that dental professionals can use daily in discussions with patients and colleagues, reflecting the exponential growth of our understanding of the processes involved in periodontal disease.
A pivotal discovery was the role of the body’s immunoinflammatory response in perio tissue destruction. Understanding that periodontal disease development and progression are the result of the interplay between the perio pathogens and the body’s response changed everything. Another seismic shift occurred when the central importance of patients’ DNA in the progression of gingivitis to periodontitis was realized.
We now know that a very small proportion of all bacterial species in the mouth can cause periodontitis. Perio pathogens represent less than 5% of the 700 or so species of bacteria in the oral cavity. Oral biofilms are mixed microorganism colonies whose growth occurs in a precise sequential manner.
The early colonizing bacteria in a newly forming biofilm are not pathogenic. They cannot cause periodontitis, but they can cause gingivitis. As the undisturbed biofilm continues to grow, perio pathogens come on board as later colonizers and become the predominant species.
It is important to understand that oral bacteria get inside gingival epithelial cells. They can migrate from cell to cell and reach the underlying connective tissue. When this occurs, the body responds by mobilizing the inflammatory response to combat the bacterial invasion. The primary physical properties of the inflammatory response are blood vessel dilation in the area of gingival tissue involvement, and the opening of gaps in the blood vessel walls.
Increased vasodilation brings more blood to the affected area, and increased vascular permeability allows larger immune system cells to pass through to the tissue. Blood flows through the gaps into the tissue, carrying the inflammatory mediators with it, increasing the volume in the tissue, which manifests clinically as redness, swelling, and bleeding, the classic signs of gingivitis.
Gingivitis is currently accepted and addressed as an early form of periodontitis. We now know that this is not really true. It is, more accurately, the gatekeeper to periodontitis. We know this from several lines of evidence. From a clinical standpoint, we know that progression from gingivitis to periodontitis is not automatic, does not occur in every patient, or in every site.
Evidence from the immune system response reveals that gingivitis is primarily a T cell response, while periodontitis is primarily a B cell and plasma cell response. Evidence that gingivitis is the gatekeeper to periodontitis also comes from a genetic standpoint. Periodontitis occurs only in genetically susceptible individuals. Disease progression requires the activation of an inflammatory switch in the form of gingivitis.
The switch turns on the patient’s DNA, if there is any to ignite. Next, the shift from T cells to B and plasma cells occurs making the tissue more susceptible to secondary infection by perio pathogens, which are programmed to take advantage of these immunocompromised tissue conditions, resulting in periodontitis.
Clinically, the initial immune system response to the perio pathogens occurs in the gingival soft tissue. If uncontrolled, the inflammatory response physically travels through the gingiva toward the alveolar bone. If it reaches the bone, or very close to it, bone resorption will occur, thus establishing full-blown periodontitis. In other words, failure to confine the inflammatory response to the gingiva results in progression to periodontitis in genetically susceptible individuals.
Decades ago, the prevailing theories about periodontal disease influenced treatment modalities. Today, our understanding of perio disease development and progression has a similar impact on current treatment modalities. Regenerative procedures challenge generally accepted ideas of the irreversibility of periodontitis. Salivary DNA testing, current and soon-to-arrive host response tools, and our understanding of the oral/systemic links are all the result of research advances.
No doubt future discoveries will have a profound effect on knowledge, and the manner in which we address periodontal disease, perhaps culminating in a cure.
Dr. Richard Nagelberg has practiced general dentistry in suburban Philadelphia for more than 27 years. He is a speaker, advisory board member, consultant, and key opinion leader for several dental companies and organizations. He lectures extensively on a variety of topics centered on understanding the impact dental professionals have beyond the oral cavity. Contact him at gr82th@aol.com.
Dental Economics and DentistryIQ Topic and Resource Categories:












 Una mesa redonda dedicada al desarrollo de programa cubano de detección del cáncer bucal centrará los debates de la Convención Internacional Estomatología 2010, que concluye en La Habana, señala Prensa Latina. La agenda científica del evento, inaugurado el pasado miércoles en el Palacio de Convenciones de La Habana, incluye además conferencias magistrales sobre la estomatología en situaciones de desastre, implantología, así como la atención estomatológica en la comunidad.
Una mesa redonda dedicada al desarrollo de programa cubano de detección del cáncer bucal centrará los debates de la Convención Internacional Estomatología 2010, que concluye en La Habana, señala Prensa Latina. La agenda científica del evento, inaugurado el pasado miércoles en el Palacio de Convenciones de La Habana, incluye además conferencias magistrales sobre la estomatología en situaciones de desastre, implantología, así como la atención estomatológica en la comunidad. Los resultados preliminares del tratamiento con células madre de las enfermedades periodontales, presentados ayer en la Convención Internacional de Estomatología que se celebra en La Habana, fueron calificados por investigadores como "muy alentadores".
Los resultados preliminares del tratamiento con células madre de las enfermedades periodontales, presentados ayer en la Convención Internacional de Estomatología que se celebra en La Habana, fueron calificados por investigadores como "muy alentadores".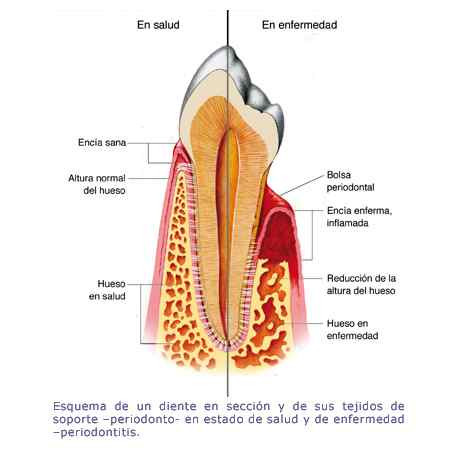 En las 24 unidades de medicina familiar del IMSS en Yucatán, se otorga un promedio de 17 mil 635 consultas por este padecimiento; el 65 por ciento de los casos corresponde a pacientes del sexo femenino y el 34 por ciento al masculino; siendo el grupo de edad con mayor prevalencia el de 50 a 59 años de edad, con 32 por ciento del total.
En las 24 unidades de medicina familiar del IMSS en Yucatán, se otorga un promedio de 17 mil 635 consultas por este padecimiento; el 65 por ciento de los casos corresponde a pacientes del sexo femenino y el 34 por ciento al masculino; siendo el grupo de edad con mayor prevalencia el de 50 a 59 años de edad, con 32 por ciento del total.
